


TuberCurea – Hepatoprotective Herbal Capsules
Innovator ID
SNDMAC025
Standardized herbal capsule that protects the liver during TB treatment, improves adherence, reduces side effects, and supports successful recovery.
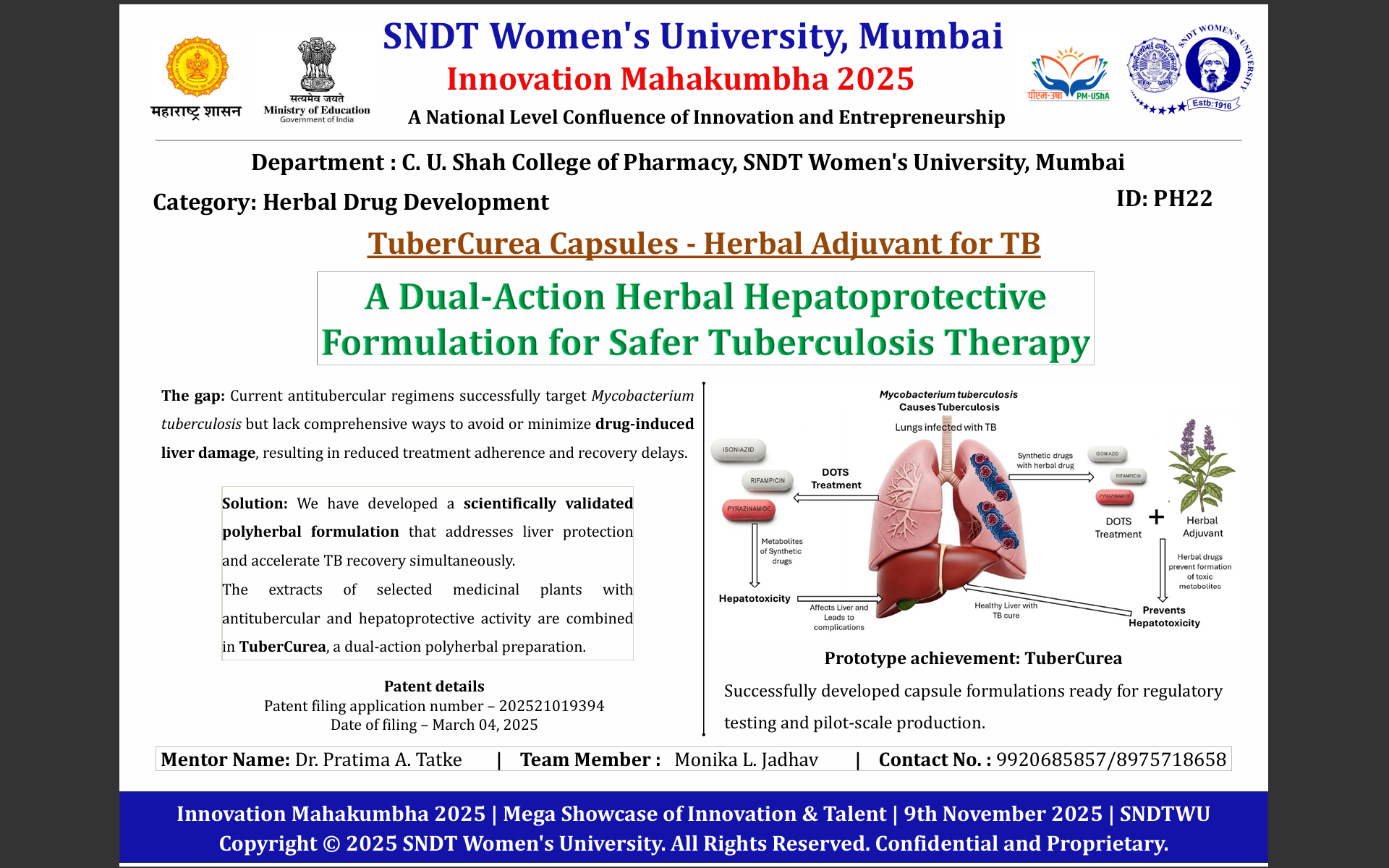
Tuberculosis patients undergoing long-term treatment frequently suffer from liver damage caused by standard anti-TB drugs such as isoniazid, rifampicin, and pyrazinamide. This drug-induced hepatotoxicity leads to treatment interruption, poor adherence, prolonged recovery, and contributes to multidrug resistance. Currently, there is no safe, standardized, and affordable hepatoprotective formulation specifically designed to support TB therapy. Patients require a solution that protects the liver while allowing continuation of essential anti-TB medication, improving treatment outcomes and quality of life.
TB treatment centers, government TB control programs, hospitals, AYUSH practitioners, pharmaceutical manufacturers, NGOs working in public health, and patients undergoing long-term TB therapy who require liver protection to maintain adherence and avoid treatment-related side effects.

TB treatment centers, government TB control programs, hospitals, AYUSH practitioners, pharmaceutical manufacturers, NGOs working in public health, and patients undergoing long-term TB therapy who require liver protection to maintain adherence and avoid treatment-related side effects.
TuberCurea prevents liver damage during TB treatment, allowing patients to safely continue essential medication without interruption. This reduces hospitalization costs, treatment failure, and relapse rates. Healthcare institutions benefit from improved patient outcomes, higher adherence, and reduced drug resistance rates. The formulation is scientifically validated, standardized using modern analytical methods, and aligns with the increasing integration of herbal therapeutics into mainstream care. Its dual-action benefit and regulatory readiness make it a high-value, clinically important solution.
We Would Require Support In the Following :
Technical and Product Development Support; Mentorship and Expert Guidance; Funding and Financial Assistance; Market Research and Business Planning Support; Intellectual Property (IP) and Legal Support; Networking, Collaboration, and Investor Connect
Capital to achieve the desired outcome, we would require
₹25–50 L, partial plan
And would be required for
Prototype Development Support; Research, Design & Validation Funding; Intellectual Property (IP) Protection Assistance; Business Model & Market Access Support; Pilot Testing and Field Trial Support
Expected Return Should Be in:
Less than 3 years